[Structural Features]
The structural formula of silane coupling agent is Y-R-Si-X3, where Y represents an organic functional group, R represents an alkyl group, and X represents a group that can be hydrolyzed. Y mainly reacts with organic polymers, while the hydrolyzable group X mainly controls the hydrolysis rate. Under the same hydrolysis conditions, the hydrolysis rate of large functional groups is slow; In acidic environments, hydrolysis of longer alkyl groups is slower. For example, the hydrolyzable alkoxy group is usually ethoxy or methoxy. Under the same hydrolysis conditions, the hydrolysis rate of trimethoxysilane is faster than that of triethoxysilane. The hydrolysis rate of α - methacryloyloxy-methyl triethoxysilane in acidic solution is 20 times that of γ - methacryloyloxy-propyl triethoxysilane.
[Mechanism of Action]
There are various explanations for the interfacial interaction mechanism of silane coupling agents between two materials with different properties, such as chemical bond theory, reversible equilibrium theory, and physical adsorption theory. However, interface phenomena are very complex, and a single theory is often difficult to fully explain. In general, chemical bonding theory can better explain the interaction between silane coupling agents and inorganic materials. According to this theory, the coupling process of silane coupling agents at different material interfaces is a complex liquid-solid surface physicochemical process. Firstly, silane coupling agents have low viscosity and surface tension, high wetting ability, and small contact angles with glass, ceramic, and metal surfaces. They can quickly spread out on their surfaces, allowing inorganic materials to be wetted by silane coupling agents; Secondly, once the silane coupling agent spreads on its surface, the material surface is wetted, and the two functional groups on the silane coupling agent molecules diffuse towards surfaces with similar polarity. Due to the thin water layer adsorbed on the surface of the material in the atmosphere, the alkoxy group at one end is hydrolyzed into silanol group, which is oriented towards the inorganic material surface and undergoes hydrolysis condensation reaction with the hydroxyl group on the material surface; Organic functional groups are oriented towards the surface of organic materials, and during crosslinking and solidification, they undergo chemical reactions to complete the coupling process between heterogeneous materials. The brief equation for a chemical reaction is as follows:
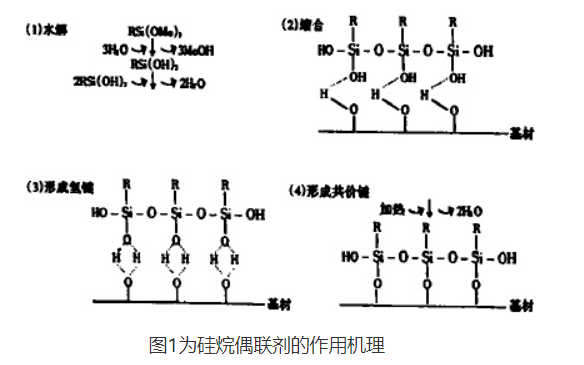
[Type]
1. Organosilicon peroxide coupling agent. Organosilicon peroxide coupling agent is also a type of coupling agent that has been studied in recent years. It differs from traditional coupling agents in that the hydrolyzable group X is an OOR group. Its characteristic is that the peroxide group is easily decomposed into highly reactive free radicals when heated. It can not only serve as a coupling agent between organic and inorganic compounds, but also couple two identical or different organic compounds. It can also couple with non-polar organic compounds such as polyolefins and silicone rubber. This effectively solves the two major problems of ordinary organic silicon coupling agents, and the more valuable aspect is that organic silicon peroxide coupling agents have fast curing speed and high adhesive strength, thereby expanding the applicability of organic silicon peroxide coupling agents.
2. The main silane coupling agent currently used both domestically and internationally is the gamma functional silane, which is separated by three methylene groups between the silicon atom and the organic functional group. Organic silicon compounds with this structure have good stability.
3. Long chain alkyl silane coupling agent Long chain alkyl alkoxysilane is a new type of organic silicon compound with a structural formula of YRnSiX3- n, where n=0~3, X is usually - OCH3 and - OC2H5, and Y is a long-chain alkyl group. The representative product is DH-109, with a chemical name of methyldodecyldimethoxysilane and a structural formula of C12H25CH3Si (OCH3) 2.
4. The commonly used silane coupling agent for bifunctional groups is the trialkoxy type, but trialkoxy type coupling agents may reduce the stability of the matrix resin.
5. The synthesis of new polymer coupling agents with active silane groups is also one of the development directions of silane coupling agents. This type of coupling agent has better compatibility with the resin in adhesives and can form a uniform surface on the surface of the adhesive, thus having better bonding effect. Japanese company NUC has developed a new type of polymer coupling agent (MMCA), which is a polymer compound with basic functions of silane coupling agent and various organic functional groups on the main chain of polysiloxane. MMCA not only serves as a bonding agent for inorganic organic interfaces, but also endows composite materials with heat resistance, wear resistance, chemical resistance, impact resistance, and hydrophobicity.
6. Starting from A-1100 developed by UCC, modified amino silane coupling agents can be derived into diaminosilanes containing one primary and one secondary amino group (A-1120), triaminosilanes containing one 11 primary and two secondary amino groups (A-5162), and polyaminosilanes containing one primary and multiple secondary amino groups (Y-5691), among others. These silanes containing free amino groups have high alkalinity and reactivity, and with the increase of amino groups, the flexural strength of plastic products also increases accordingly
【 Application 】
1. Used as a surface treatment agent mainly for the surface treatment of glass fibers, it can improve the bonding performance between glass fibers and resins, greatly enhancing the strength, electrical, water resistance, weather resistance and other properties of glass fiber reinforced composite materials; Even in the wet state, it can significantly improve the mechanical properties of composite materials.
2. Used for filling plastics with inorganic fillers, the fillers can be surface treated in advance or directly added to the material. It can improve the dispersibility and adhesion of fillers in resins, enhance process performance, and improve the mechanical, electrical, and weather resistance properties of filled plastics (including rubber).
3. As a thickening agent for sealants, adhesives, and coatings, it can improve the bonding strength, water resistance, high temperature resistance, weather resistance, and other properties of sealants, adhesives, and coatings.
4. Used as an adhesion promoter for difficult to bond materials such as polyolefins (PE, PP) and specialty rubbers (such as silicone rubber, EPR, CR, fluororubber).
5. It can be used as a textile auxiliary and organic silicon lotion to improve the wearing performance of trade textiles. The test fabric has the advantages of softness, fullness, good resilience, wrinkle resistance, antistatic, waterproof, washable, comfortable wearing, etc.
6. Silane coupling agents are important raw materials for preparing silicone resin solid trypsin carriers in biochemical and environmental aspects. And it can make the solidifying enzyme insoluble in water, and the inactive solid-phase enzyme can continue to be used after filtration, which not only improves the utilization rate of biological enzymes, but also avoids pollution and waste.
7. Used for the formation of "dentures" in domestic dentistry, "dentures" are generally made of modified methacrylate polymers and fillers. To improve the adhesion between them and enhance the strength and stiffness of the "dentures", KH-570 silane coupling agent is added to the formula before it solidifies. In addition, silane coupling agents are widely used as waterproofing agents, crosslinking agents, metal preservatives, protective agents for glass and ceramics, finishing agents for fibers and leather, and additives for petroleum development and transportation. Some coupling agents (such as - aminopropyltriethoxysilane, - chloropropyltriethoxysilane) can also be used as combustion aids when exposed to open flames.
[Application Effect]
1. Application effect of coupling agent in rubber
(1) When using nanomaterials as rubber reinforcing agents for the interaction between rubber and fillers, due to the fact that nanoparticles are prepared under non-equilibrium and harsh conditions, their surface atoms are highly activated and their surface energy is high, making it easy for nanoparticles to aggregate into clusters. In addition, the surface properties of nanoparticles and their low dispersion energy result in poor compatibility with rubber. After treatment with coupling agents, nano white carbon black has lower surface energy and is easily infiltrated by rubber macromolecules, which improves the dispersion of white carbon black fillers. At the same time, due to the bridging effect of coupling agents between rubber and fillers, the interface bonding between nano white carbon black particles and rubber matrix is enhanced, improving its reinforcement ability on rubber matrix.
(2) The effect on the vulcanization characteristics of rubber: Adding coupling agents during the rubber vulcanization process can improve the vulcanization characteristics of rubber materials, significantly improving the processing and mechanical properties of rubber products.
(3) The influence of silane coupling agent on the physical and mechanical properties of rubber materials. Silane coupling agent plays a coupling and filling role in the system. When the amount of coupling agent is small, the rubber macromolecules are less bound, easy to slide and orient, and the stress distribution is uniform, resulting in higher tensile strength; As the amount of coupling agent increases, the number of coupling agent molecules increases, and the rubber macromolecules are more tightly bound and less prone to sliding, resulting in uneven stress distribution and a decrease in tensile strength; The dosage of coupling agent continues to increase, and excessive coupling agent is filled into the system, making the macromolecular chains easy to slide and orient, resulting in uniform stress distribution and an increase in tensile strength. Silane coupling agents can also improve the physical processing properties of filled rubber. Due to the improvement of compatibility and dispersibility between fillers and base adhesives by silane coupling agents, the viscosity of the adhesive is reduced, mixing time is shortened, extrusion processing performance is improved, and product quality is enhanced.
2. Application of silane coupling agent in plastics
(1) Used as a raw material to synthesize organosilicon plastics, methyl or phenylsilane is used as a monomer to hydrolyze and condense to form organosilicon resin, which is then compressed or layered with fillers such as mica, asbestos, glass fiber or glass cloth to form thermosetting organosilicon plastics. It has high heat resistance, excellent electrical insulation and arc resistance, as well as waterproof, moisture-proof and other properties.
(2) The water crosslinked polyolefins and moisture cured acrylic organic silicon coatings sold in the modified plastics and polymer market utilize the property of trialkoxymethyl alkyl breaking down and self condensing upon contact with water to form siloxane bonds. In addition, 4-aminophenyltrimethoxysilane modified by Japan's Hoso Corporation has been used to produce low thermal expansion and adhesive polyimides. Dow Corning Corporation in the United States has used aminophenoxypropyltrimethoxysilane to produce polyvinyl acetate adhesives containing alkoxysilane.
(3) Used as a coupling agent for composite materials
1) The application of fiberglass reinforced plastic (FRP) FRP is made by coating unsaturated polyester or phenolic resin with fiberglass or fiberglass cloth and laminating and curing it. The fiberglass or fiberglass cloth used is generally treated with silane coupling agent to improve the wet mechanical and electrical properties of FRP, reduce water absorption rate, and improve appearance.
2) The application of silane coupling agents in mineral filled thermosetting plastics can improve the dispersibility and adhesion properties of inorganic fillers in resins. Using silane coupling agent as a surface treatment agent for fillers, the dispersibility and adhesion of fillers in phenolic resin are fully improved, and the rigidity, bending resistance, tensile strength, and other properties of grinding wheels are enhanced.
3) The application of non reactive functional groups such as polyethylene and polypropylene in mineral filled thermoplastic materials and the use of coupling agents are generally not significant. However, adding certain free radical generating additives to composite materials can improve the physical properties of most thermoplastic materials containing inorganic fillers and the dispersibility of pigments in the plastic, as well as protect the plastic from water erosion when immersed in water. Meanwhile, for glass fiber reinforced thermoplastic resins, silane coupling agents endow properties far superior to glass, and can maintain good physical and electrical properties when the mixture is under harmful conditions. Mixing silane coupling agents with mineral fillers can improve the physical and wet electrical properties of the filling system, making it close to or in some cases surpassing non filling resins.